Posts by Marc Sanchez
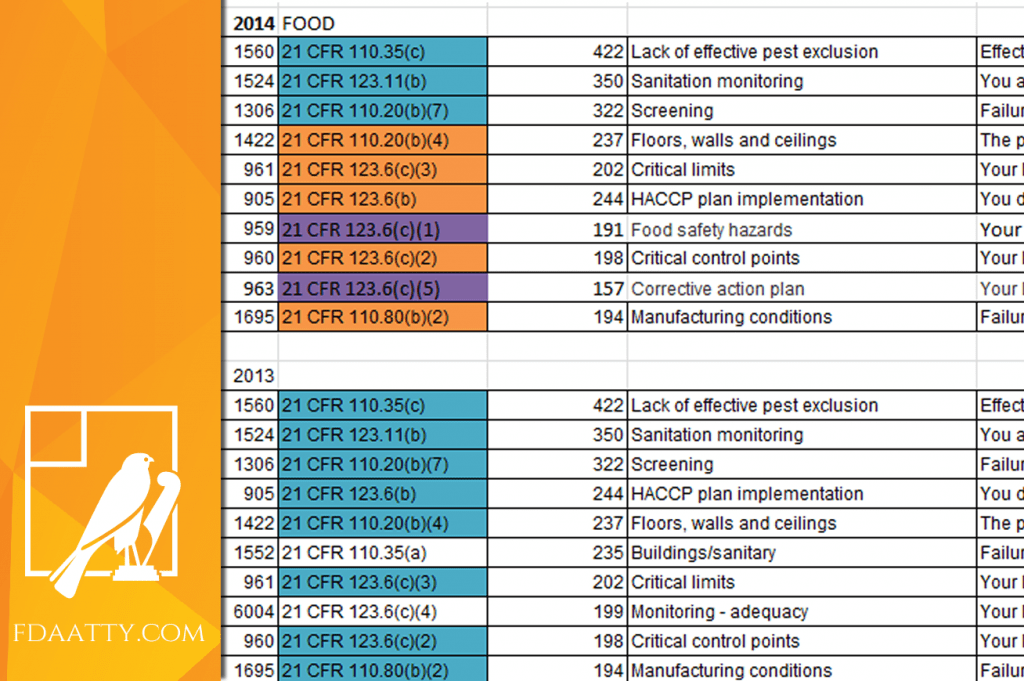
Review: FY 2014 Inspectional Observation Summaries
At the end of each fiscal year the US FDA posts a summary of the Inspectional Observations from Form 483. The fiscal year for the FDA begins October 1 and concludes on September 30. The fiscal year summary provides an excellent opportunity to review the enforcement activities and interests of the FDA. In comparing the…
Read MoreFDA, FSMA, and the USDA Budget Increases- Update from Latest Spending Bill
The latest reporting on the spending bill passed by the United States House of Representatives contains budget increases for the U.S. Food and Drug Administration (FDA) and the USDA Food Safety Inspection Services (FSIS). The Washington Post this morning reports the following on the two Agency’s budgets: There’s $2.589 billion for the Food and…
Read MoreFTC Sues Gerber Over Unapproved Health Claim
The US Federal Trade Commission announced today its intent to sue Gerber for unapproved and unsubstantiated health claims made on its
Read MoreFDA Textbook by Springer International; Release Date Annouced
Gluten Free Label Rule Now In Effect
Last year the US Food and Drug Administration (FDA) annouced a new rule for gluten free labeling. The rule gave manufacturers until yesterday (August 5, 2014) to update their labels. Any labels not in conformnace with the new rule will be deemed misbranded. More from the US FDA press release: In August 2013, the Food…
Read MoreThree Steps to Selling FDA Approved Products on Amazon
“Is it FDA approved?” This is the most frequent question received by phone and e-mail. It’s also the most commonly asked question by Amazon. The online retailer’s popularity with start-up companies is surging. It allows a quick and convenient means to sell and distribute products across the U.S. Browsing Amazon one will find a range…
Read MorePress Release: Springer Taps Marc Sanchez for Food Law Textbook
For Immediate Release: June 23, 2014 Marc C. Sanchez, Esq. Senior Counsel, Contract In-House Counsel and Consulting, LLC Adjunct Professor of Law, Northeastern University 404.895.4882 msanchez@fdaatty.com Springer International Taps Marc Sanchez for New Food Law Title Atlanta, GA: Springer International and Attorney and Professor Marc Sanchez announced a new title to…
Read MoreFDA Finalizes Rule Prohibiting Certain Nutrient Content Claims for DHA, EPA, and ALA Omega-3 Fatty Acids
The FDA annouced a new rule in late April limiting the claims that can be made about food and dietary supplement products containing Omega 3 Fatty Acids. Nutrient content claims are claims about the nutrients in an ingredient. They include statements like “high in” or “excellent source of” among others. The full statement from the…
Read MoreAppeals & FDA Meetings: Insights from HQ Visit
An enforcement meeting with the US FDA, whether in person or over the phone, can become an intimidating task. There are number of variables to balance: process, terms, regulations and statutes, and personalities. All of this on top of the administrative check-list that accompanies any interaction with a Governmental agency. I could think of no…
Read MoreWatermelon Water – New Fad, but FDA Approved?
ABC news and other media outlets are reporting on a new beverage fad – watermelon water. Before jumping on the bandwagon, it is important to ask is this FDA approved? The FDA offers guidance and regulations which stipulate when a beverage is a conventional food item and when it is a dietary supplement. This distinction…
Read MoreData Integrity and Form 483 or Warning Letters
Data integrity is an essential component of both clinical and nonclinical research conducted under the supervision of the Food and Drug Administration.
Read MoreNew FDA E-Cigarette Rules – What Does It Mean
Yesterday (April 24, 2014) the US Food and Drug Administration (FDA) announced new regulation of e-cigarettes. The rule changes the FDA’s prior position of only regulating e-cigarettes if the aim of the product was to aide in smoking cessation. Effectively the prior regulations intended to regulate e-cigarettes only if they were intended to be marketed…
Read More