Review: FY 2014 Inspectional Observation Summaries
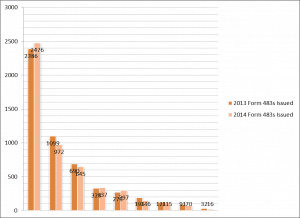
At the end of each fiscal year the US FDA posts a summary of the Inspectional Observations from Form 483. The fiscal year for the FDA begins October 1 and concludes on September 30. The fiscal year summary provides an excellent opportunity to review the enforcement activities and interests of the FDA. In comparing the observations made from fiscal year to fiscal year trends emerge that allow facilities to prepare for a facility inspection. This year, as with most, the top ten observations remain largely unchanged. FY 2014 did note a marked increase in the number of food facility inspections as can be seen in the graph below.
As can be seen below the top ten observations deviate little from year to year. This provides new and existing facilities a head start to FY 2015 as they consider a FDA inspection. Namely by reviewing the top ten observations for compliance. This small step will greatly improve a facility’s experience during an inspection.
Please contact FDA Atty for additional statistics, such as with veterinary products (food and drugs), and with any questions.
Comparison of Total Observations for FY 2014
Comparison of Top Ten Observations – FY 2013 to FY 2014
Key: Blue = Observation Listed
Are you in trouble with the FDA?
Don’t panic — you’ve got backup. Download 5 Tips to Help You Navigate FDA Enforcement and learn how to resolve the situation right now.